- Преподавателю
- Иностранные языки
- Рабочая тетрадь по сварочному производству
Рабочая тетрадь по сварочному производству
| Раздел | Иностранные языки |
| Класс | - |
| Тип | Другие методич. материалы |
| Автор | Кузовлева Н.Н. |
| Дата | 09.02.2016 |
| Формат | doc |
| Изображения | Есть |
 Министерство образования и науки Российской Федерации
Министерство образования и науки Российской Федерации
Федеральное государственное бюджетное образовательное учреждение
высшего профессионального образования
«Магнитогорский государственный технический университет
им. Г. И. Носова»
Многопрофильный колледж
Английский язык
Профессиональный модуль
Рабочая тетрадь для специальности 44.02.06 Профессиональное обучение. Сварочное производство
Магнитогорск
2016
Рецензент:
Михайлова Е.А., преподаватель МпК,
Симонова Г.К., директор «Английский акцент»
Английский язык. Рабочая тетрадь для специальности 44.02.06 «Профессиональное обучение. Сварочное производство».
Автор-составитель: Кузовлева Н.Н. преподаватель МпК
Рабочая тетрадь составлена в соответствии с рабочей программой по учебной дисциплине и предназначена для студентов очного обучения специальности 44.02.06 « Профессиональное обучение. Сварочное производство».
Рабочая тетрадь рассчитана на аудиторную работу под руководством преподавателя, либо на самостоятельную работу.
Содержание
Введение…………………………………………………3
Metals 4
Properties of Metals ……………………………………………………….7
Steel .8
Methods of Steel Heat Treatment .9
Metalworking and Metal Properties 11
Mechanical Properties of Materials(Part I) 13
Mechanical Properties of Materials(Part II) 16
Welding 18
Other Types of Welding 21
Types of Corrosion ………………………………………23
Глоссарий 27
Литература .30
Введение
Целью рабочей тетради является формирование элементарных навыков устной речи по специальности студентов и чтения литературы по данной специальности на английском языке для извлечения необходимой информации.
Рабочая тетрадь состоит из профессионально - ориентированных текстов, глоссария по сварочному производству.
Тексты предназначены для чтения и развития элементарных навыков устной речи по специальности студентов. Англо-русский терминологический словарь-минимум позволяет найти точное значение термина, устраняя необходимость пользоваться большими специализированными словарями, облегчая понимание текста и ускоряя процесс обучения.
Послетекстовые упражнения служат для закрепления изученного материала и являются контрольно-обучающими.
METALS
Metals are materials most widely used in industry because of their properties. The study of the production and properties of metals is known as metallurgy.
The separation between the atoms in metals is small, so most metals are dense. The atoms are arranged regularly and can slide ever each other. That is why metals are malleable (can be deformed and bent without fracture) and ductile (can be drawn into wire). Metals vary greatly in their properties. For example, lead is soft and can be bent by hand, while iron can only be worked by hammering at red heat.
The regular arrangement of atoms in metals gives them a crystalline structure. Irregular crystals are called grains. The properties of the metals depend on the size, shape, orientation, and composition of these grains. In general, a metal with small grains will be harder and stronger than one with coarse grains.
Heat treatment such as quenching, tempering, or annealing controls the nature of the grains and their size in the metal. Small amounts of other metals (less than 48 per cent) are often added to a pure metal. This is called alloying (легирование) and it changes the grain structure and properties of metals.
All metals can be formed by drawing, rolling, hammering and extrusion, but some require hot-working. Metals are subject to metal fatigue and to creep (the slow increase in length under stress) causing deformation and failure. Both effects are taken into account by engineers when designing, for example, airplanes, gas-turbines, and pressure vessels for high-temperature chemical processes. Metals can be worked using machine-tools such as lathe, milling machine, sharper and grinder.
The ways of working a metal depend on its properties. Many metals can be melted and cast in moulds, but special conditions are required for metals that react with air.
Vocabulary:
property ['propəti] -свойство
metallurgy [mə'tælədʒi] -металлургия
separation [sepə'rei∫ən] - разделение, отстояние
dense [dens] - плотный
arrangement [ə'reindʒmənt] - pacположение
regularly ['regjuləli] - регулярно, правильно
to slide [slaid] - скользить
malleable ['mæliəbl] - ковкий, податливый, способный деформироваться
bent [bent] pp of bend - гнуть
to fracture ['frækt∫ə] - ломать
ductile [dΛk'tail] - эластичный, ковкий
to draw [drɔ:] - волочить, тянуть
wire ['waiə] - проволока
lead [led] - свиинец
iron ['aiən] - железо, чугун
grain [grein] - зерно
to depend [di'pend] - зависеть
size [saiz] - размер, величина
shape [∫eip] - форма, формировать
composition [kompə'zi∫n] - состав
coarse [kɔ:s] - грубый, крупный
treatment ['tri:tmənt] - обработка
quenching [kwent∫iŋ] - закалка
tempering ['tempəriŋ] - отпуск после закалки, нормализация
annealing [ə'niliŋ] - отжиг, отпуск
rolling ['rəuliŋ] - прокатка
to hammer ['hæmə] - ковать (напр, молотом)
extrusion [ekstr'uʒn] - экструзия
metal fatigue [fə'ti:gju] - усталость металла
creep [kri:p] - ползучесть
stress [stres] - давление, напряжение
failure ['feiljə] - повреждение, разрушение
vessel [vesl] - сосуд, котел, судно
lathe [Ieið] - токарный станок
milling machine [mi'liŋ] - фрезерный станок
shaper [∫eipə] - строгальный станок
grinder [graində] - шлифовальный станок
to melt [melt] - плавить, плавиться расплавить
to cast [ka:st] - отливать, отлить
mould [məuld] - форма (для отливки)
General understanding:
-
What are metals and what do we call metallurgy?
-
Why are most metals dense?
-
Why are metals malleable?
-
What is malleability?
-
What are grains?
-
What is alloying?
-
What is crystalline structure?
-
What do the properties of metals depend on?
-
What changes the size of grains in metals?
-
What are the main processes of metal forming?
-
How are metals worked?
-
What is creeping?
Exercise 1. Find the following words and word combinations in the text:
-
Свойства металлов
-
расстояние между атомами
-
правильное расположение
-
сильно отличаются по своим свойствам
-
кристаллическая структура
-
размер зерен
-
форма зерен
-
8. закалка
9. отжиг
10. волочение
11. прокатка
12.ковка
13.экструзия
-
структура и свойства зерна
-
горячая обработка
-
усталость металла
-
ползучесть металла
-
плавка и отливка в формы
-
способы обработки металлов
Exercise 2. Complete the following sentences:
-
Metals are...
-
Metallurgy is...
-
Most metals are...
-
The regular arrangement of atoms in metals...
-
Irregular crystals...
-
The properties of the metals depend...
-
Metals with small grains will be...
8. ...controls the nature of the grains in the metal.
9. Alloying is...
-
All metals can be formed by...
-
Creep is...
12. Metals can be worked using...
Exercise 3. Translate into English:
-
Металлы - плотные материалы потому, что между атомами в металлах малое расстояние.
-
Металлы имеют кристаллическую структуру из-за правильного расположения атомов.
-
Чем меньше зерна, тем тверже металл.
-
Закалка и отжиг изменяют форму и размер зерен в металлах.
-
Легирование изменяет структуру зерен и свойства металлов.
-
Металл деформируется и разрушается из-за усталости и ползучести.
PROPERTIES OF METALS
The metals resemble one another in their general chemical behaviour with other substances, but they differ markedly in activity, the uses to which metals are based upon their physical or chemical properties.
The metals vary greatly in density. The lightest is lithium, which has the density of 0.534 and is, therefore, about one-half as heavy as water. The heaviest is osmium (d.22.48) which is closely related to platinum (d. 21.45) in physical and chemical properties. The so-called light metals, of which sodium, potassium, magnesium and aluminium are examples, have a density less than 4; iron lead, tin, silver, etc. are known as heavy metals.
The metals also vary in hardness, from potassium, which can be melded like wax, to chromium, which cut glass. The metals and other substances differ in the extent to which they can resist a strain that tends to bring about a permanent change in their form. All substances offer more or less resistance to the flow of an electric current through them. With any given substance, its dimensions and the temperature determine the resistance.
The solids obtained when two or more metals are mixed in the molten condition and allowed to solidity are called alloys. Each consistent of an alloy is called a component. Alloys may be binary (two-component), ternary (three-component), etc. the ability of various metals to form alloys differs greatly.
Vocabulary:
chemical properties['kemikel 'prɔpətiz] - химические свойства
density['densiti] - плотность
permanent change ['pə:mənənt t∫ein(d)ʒ] - постоянное изменение
dimension [dai'men∫n] - размер
resistance [ri'zist(ə)ns] - сопротивление, устойчивость.
General understanding:
-
How do the metals resemble one another?
-
How do the metals vary?
-
How do we call each consistent of an alloy?
Exercise 1. Complete the following sentences:
The metals vary greatly in … .
The metals and other substances differ in … .
-
Alloys may be binary (two-component) … .
STEEL
The most important metal in industry is iron and its alloy - steel. Steel is an alloy of iron and carbon. It is strong and stiff, but corrodes easily through rusting, although stainless and other special steels resist corrosion. The amount of carbon in steel influences its properties considerably. Steels of low carbon content (mild steels) are quite ductile and are used in the manufacture of sheet iron, wire, and pipes. Medium-carbon steels containing from 0.2 to 0.4 per cent carbon are tougher and stronger and are used as structural steels. Both mild and medium-carbon steels are suitable for forging and welding. High-carbon steels contain from 0.4 to 1.5 per cent carbon, are hard and brittle and are used in cutting tools, surgical instruments, razor blades and springs. Tool steel, also called silver steel, contains about 1 per cent carbon and is strengthened and toughened by quenching and tempering.
The inclusion of other elements affects the properties of the steel. Manganese gives extra strength and toughness. Steel containing 4 per cent silicon is used for transformer cores or electromagnets because it has large grains acting like small magnets. The addition of chromium gives extra strength and corrosion resistance, so we can get rust-proof steels. Heating in the presence of carbon or nitrogen-rich materials is used to form a hard surface on steel (case-hardening). High-speed steels, which are extremely important in machine-tools, contain chromium and tungsten plus smaller amounts of vanadium, molybdenum and other metals.
Vocabulary:
alloy ['ælɒi] - сплав
carbon ['ka:bən] -углерод
stiff [stif] - жесткий
to corrode [kə'reud] - разъедать, ржаветь
rusty ['rΛsti] - ржавый
stainless - нержавеющий
to resist [ri'zist] - сопротивляться
considerably [kən'sidərəbli] - значительно, гораздо
tough [tΛf] - крепкий, жесткий, прочный, выносливый
forging ['fo:dʒiŋ] - ковка
welding ['weldiŋ] - сварка
brittle ['britl] - хрупкий, ломкий
cutting tools - режущие инструменты
surgical instruments [sə:dʒikl] - хирургические инструменты
blade [bleid] - лезвие
spring [spriŋ] - пружина
inclusion [in'klʒuən] - включение
to affect [ə'fekt] - влиять
manganese [mæŋgə'ni;z] - марганец
silicon ['silikən] - кремний
rust-proof - нержавеющий
nitrogen ['naitrədʒən] - азот
tungsten [tΛŋstən] - вольфрам
General understanding;
-
What is steel?
-
What are the main properties of steel?
-
What are the drawbacks of steel?
-
What kinds of steel do you know? Where are they used?
-
What gives the addition of manganese, silicon and chromium to steel?
-
What can be made of mild steels (medium-carbon steels, high-carbon steels)?
-
What kind of steels can be forged and welded?
-
How can we get rust-proof (stainless) steel?
9. What is used to form a hard surface on steel?
10. What are high-speed steels alloyed with
Exercise 1. Find the following words and word combinations in the text:
-
сплав железа и углерода
-
прочный и жесткий
-
легко коррозирует
-
нержавеющая сталь
-
низкое содержание углерода
-
ковкость
-
листовое железо, проволока, трубы
-
конструкционные стали
-
пригодны для ковки и сварки
-
твердый и хрупкий
-
режущие инструменты
-
хирургические инструменты
-
инструментальная сталь
-
упрочнять
15. добавление марганца (кремния, хрома, вольфрама, молибдена, ванадия)
METHODS OF STEEL HEAT TREATMENT
Quenching is a heat treatment when metal at a high temperature is rapidly cooled by immersion in water or oil. Quenching makes steel harder and more brittle, with small grains structure.
Tempering is a heat treatment applied to steel and certain alloys. Hardened steel after quenching from a high temperature is too hard and brittle for many applications and is also brittle. Tempering, that is re-heating to an intermediate temperature and cooling slowly, reduces this hardness and brittleness. Tempering temperatures depend on the composition of the steel but are frequently between 100 and 650 °C. Higher temperatures usually give a softer, tougher product. The colour of the oxide film produced on the surface of the heated metal often serves as the indicator of its temperature.
Annealing is a heat treatment in which a material at high temperature is cooled slowly. After cooling the metal again becomes malleable and ductile (capable of being bent many times without cracking).
All these methods of steel heat treatment are used to obtain steels with certain mechanical properties for certain needs.
Vocabulary:
to immerse [i'mə:s] - погружать
to apply [ə'plai] - применять
intermediate [intə'mi:diət] - промежуточный
oxide film ['ɒksaid] - оксидная пленка
annealing [æ'ni:liŋ] - отжиг, отпуск
cracking - растрескивание
General understanding:
-
What can be done to obtain harder steel?
-
What makes steel more soft and tough?
-
What makes steel more malleable and ductile?
-
What can serve as the indicator of metal temperature while heating it?
-
What temperature range is used for tempering?
6. What are the methods of steel heat treatment used for?
Exercise 1. Translate into English the following words and word combinations:
-
температура нормализации
-
мелкозернистая структура
-
быстрое охлаждение
-
закаленная сталь
-
состав стали
-
окисная пленка
-
индикатор температуры
-
медленное охлаждение
METALWORKING AND METAL PROPERTIES
An important feature of hot working is that it provides the improvement of mechanical properties of metals. Hot-working {hot-rolling or hot-forging) eliminates porosity, directionality, and segregation that are usually present in metals. Hot-worked products have better ductility and toughness than the unworked casting. During the forging of a bar, the grains of the metal become greatly elongated in the direction of flow. As a result, the toughness of the metal is greatly improved in this direction and weakened in directions transverse to the flow. Good forging makes the flow lines in the finished part oriented so as to lie in the direction of maximum stress when the part is placed in service.
The ability of a metal to resist thinning and fracture during cold-working operations plays an important role in alloy selection. In operations that involve stretching, the best alloys are those which grow stronger with strain (are strain hardening) - for example, the copper-zinc alloy, brass, used for cartridges and the aluminum-magnesium alloys in beverage cans, which exhibit greater strain hardening.
Fracture of the work piece during forming can result from inner flaws in the metal. These flaws often consist of nonmetallic inclusions such as oxides or sulfides that are trapped in the metal during refining. Such inclusions can be avoided by proper manufacturing procedures.
The ability of different metals to undergo strain varies. The change of the shape after one forming operation is often limited by the tensile ductility of the metal. Metals such as copper and aluminum are more ductile in such operations than other metals.
Vocabulary:
feature ['fi:t∫ə] - черта, особенность
to provide [prə'vaid) - обеспечивать
improvement [im'pru:vmənt] - улучшение
property ['prɒpəti] - свойство
eliminate [i'limineit] - ликвидировать, исключать
porosity ['pɔ:rəsi] - пористость
directional [di'rek∫ənl] - направленный
to segregate ['segrigeit] - разделять
casting ['ka:stiŋ] - отливка
elongated [:i:lɒŋ'geitid] - удлиненный
to weaken ['wi:kn] - ослабевать, ослаблять
transverse ['trænzvɜ:s] - поперечный
flow [fləu] - течение, поток
finished ['f ini∫t] - отделанный
thinning - утончение
fracture ['frækt∫ə] - разрушение
strain hardening - деформационное упрочнение
brass [bra:s] - латунь
beverage ['bevəridʒ] - напиток
can [kæn] - консервная банка
to exhibit [ig'zibit] - проявлять
inner ['inə] - внутренний
flaws [flo:z] - недостатки, дефекты кристаллической решетки
inclusion [in'klu:ʒn] - включение
trapped - зд. заключенный
refining [ri'fain] - очищать, очистка
to avoid [a'vɔid] - избегать
to undergo [ʌndə'gəu] - подвергаться
tensile ductility - пластичность при растяжении
General understanding:
1. What process improves the mechanical properties of metals?
2. What new properties have hot-worked products?
3. How does the forging of a bar affect the grains of the metal? What is the result of this?
4. How are the flow lines in the forged metal oriented and how does it affect the strength of the forged part?
5. What are the best strain-hardening alloys? Where can we use them?
6. What are the inner flaws in the materials?
7. Can a metal fracture because of the inner flaw?
8. What limits the change of the shape during forming operations?
Exercise 1. Find the follow in the text:
1. важная особенность горячей обработки
2. улучшение механических свойств металла
3. необработанная отливка
4. направление максимального напряжения
5. способность сопротивляться утончению и напряжению
6. проявлять большее деформационное упрочение
7. разрушение детали при штамповке
8. внутренние дефекты в металле
9. неметаллические включения
10. способность металлов подвергаться деформации
11. ограничивается пластичностью металла при растяжении
Exercise 2. Translate into English:
1. Горячая обработка металла улучшает его механические свойства и устраняет пористость и внутренние дефекты.
2. Удлинение зерен в направлении текучести при ковке значительно улучшает прочность металла в этом направлении и уменьшает его прочность в поперечном.
3. Хорошая проковка ориентирует линии текучести в направлении максимального напряжения.
4. Деформационное упрочение металла при холодной обработке очень важно для получения металлов с улучшенными свойствами.
5. внутренние дефекты металла - это неметаллические включения типа окислов и сульфидов.
6. Изменение формы при штамповании металлических деталей ограничивается пластичностью металла при растяжении.
MECHANICAL PROPERTIES OF MATERIALS
Part I
Materials Science and Technology is the study of materials and how they can be fabricated to meet the need: of modern technology. Using the laboratory techniques and knowledge of physics, chemistry, and metallurgy scientists find new ways of using metals, plastic and other materials.
Engineers must know how materials respond to external forces, such as tension, compression, torsion, bending, and shear. All materials respond to these forces by elastic deformation. That is, the materials return their original size and form when the external force disappears. The materials may also have permanent deformation or they may fracture. The results of external forces are creep and fatigue.
Compression is a pressure causing a decrease in volume. When a material is subjected to a bending, shearing, or torsion (twisting) force, both tensile and compressive forces are simultaneously at work. When a metal bar is bent, one side of it is stretched and subjected to a tensional force, and the other side is compressed.
Tension is a pulling force; for example, the force in a cable holding a weight. Under tension, a material usually stretches, returning to its original length if the force does not exceed the material's elastic limit. Under larger tensions, the material does not return completely to its original condition, and under greater forces the material ruptures.
Fatigue is the growth of cracks under stress. It occurs when a mechanical part is subjected to a repeated or cyclic stress, such as vibration. Even when the maximum stress never exceeds the elastic limit, failure of the material can occur even after a short time. No deformation is seen during fatigue, but small localized cracks develop and propagate through the material until the remaining cross-sectional area cannot support the maximum stress of the cyclic force. Knowledge of tensile stress, elastic limits, and the resistance of materials to creep and fatigue are of basic importance in engineering.
Creep is a slow, permanent deformation that results from steady force acting on a material. Materials at high temperatures usually suffer from this deformation. The gradual loosening of bolts and the deformation of components of machines and engines are all the examples of creep. In many cases the slow deformation stops because deformation eliminates the force causing the creep. Creep extended over a long time finally leads to the rupture of the material.
Vocabulary:
bar [ba:'] - брусок, прут
completely [kəm'pli:tli] - полностью, совершенно
compression [kəm'рге∫ən] - сжатие
creep [kri:p] - ползучесть
cross-sectional area - площадь поперечного сечения
cyclic stress ['saiklik] - циклическое напряжение
decrease ['di:kri:s] - уменьшение
elastic deformation - упругая деформация
elastic limit - предел упругости
exceed [ik'si:d] - превышать
external forces [əks'tɜ:nl] - внешние силы
fatigue [fə'ti:gju:] - усталость металла
fracture ['frækt∫ə] - перелом, излом
loosen ['lu:sn] - ослаблять, расшатывать
permanent deformation ['рɜ:mənənt] - постоянная деформация
remaining [ri'meiniŋ] - оставшийся
shear [∫iə] - срез
simultaneously [siməl'teiniəsli] - одновременно
to stretch [stret∫] - растягивать
technique [tek'ni:ks] - методы
tension ['ten∫ən] - напряженность
to propagate ['propəgeit] - распространяться)
to bend [bend] - гнуть, согнуть
to extend [iks'tend] - расширять, продолжаться
to meet the needs - отвечать требованиям
to occur [ə'кɜ:] - происходить
to respond [ris'pɒnd] - отвечать реагировать
to suffer ['sʌfə] - страдать
torsion ['tɔ:∫ən] - кручение
twisting [twistirŋ] - закручивание, изгиб
volume ['volju:m] - объем, количество
rupture['rʌpt∫ə] - разрыв
General understanding:
1. What are the external forces causing the elastic deformation of materials? Describe those forces that change the form and size of materials?
2. What are the results of external forces?
-
What kinds of deformation are the combinations of tension and compression?
-
What is the result of tension? What happens if the elastic limit of material is exceeded under tension?
-
What do we call fatigue? When does it occur? What arе the results of fatigue?
-
What do we call creep? When does this type of permanent deformation take place? What are the results of сrеер?
Exercise 1. Find the following in the text:
1. отвечать требованиям современной технологии
2. используя лабораторные методы
3. новые способы использования металлов
4. сжатие, растяжение, изгиб, кручение, срез
5. возвращать первоначальный размер и форму
6. внешняя сила
7. постоянная деформация
8. уменьшение объема
9. растягивающие и сжимающие силы
10. превышать предел упругости материала
11. повторяющиеся циклические напряжения
12. разрушение материала
13. развитие и распространение мелких трещин
14. сопротивление материалов ползучести
Exercise 2. Translate into English the following sentences:
-
Упругая деформация - это реакция всех материалов на внешние силы, такие, как растяжение, сжатие, скручивание, изгиб и срез.
-
Усталость и ползучесть материалов являются результатом внешних сил.
-
Внешние силы вызывают постоянную деформацию и разрушение материала.
-
Растягивающие и сжимающие силы работают одновременно, когда мы изгибаем или скручиваем материал.
-
Растяжение материала выше предела его упругости дает постоянную деформацию или разрушение.
-
Когда деталь работает долгое время под циклическими напряжениями в ней появляются небольшие растущие трещины из-за усталости металла.
7. Ползучесть - это медленное изменение размера детали под напряжением.
MECHANICAL PROPERTIES OF MATERIALS
Part II
Density (specific weight) is the amount of mass in a unit volume. It is measured in kilograms per cubic meter. The density of water is 1000 kg/ m3 but most materials have a higher density and sink in water. Aluminium alloys, with typical densities around 2800 kg/ m3 are considerably less dense than steels, which have typical densities around 7800 kg/ m3. Density is important in any application where the material must not be heavy.
Stiffness (rigidity) is a measure of the resistance deformation such as stretching or bending. The Young modulus is a measure of the resistance to simple stretching or compression. It is the ratio of the applied force per unit area (stress) to the fractional elastic deformation (strain). Stiffness is important when a rigid structure is to be made.
Strength is the force per unit area (stress) that a material can support without failing. The units are the same as those of stiffness, MN/m2, but in this case the deformation is irreversible. The yield strength is the stress at which a material first deforms plastically. For a metal the yield strength may be less than the fracture strength, which is the stress at which it breaks. Many materials have a higher strength in compression than in tension.
Ductility is the ability of a material to deform without breaking. One of the great advantages of metals is their ability to be formed into the shape that is needed, such as car body parts. Materials that are not ductile are brittle. Ductile materials can absorb energy by deformation but brittle materials cannot.
Toughness is the resistance of a material to breaking when there is a crack in it. For a material of given toughness, the stress at which it will fail is inversely proportional to the square root of the size of the largest defect present. Toughness is different from strength: the toughest steels, for example, are different from the ones with highest tensile strength. Brittle materials have low toughness: glass can be broken along a chosen line by first scratching it with a diamond. Composites can be designed to have considerably greater toughness than their constituent materials. The example of a very tough composite is fiberglass that is very flexible and strong.
Creep resistance is the resistance to a gradual permanent change of shape, and it becomes especially important at higher temperatures. A successful research has been made in materials for machine parts that operate at high temperatures and under high tensile forces without gradually extending, for example the parts of plane engines.
Vocabulary
ability [ə'biliti] - способность
absorb [əb'so:b] - поглощать
amount [ə'maunt] - количество
application [æpli'kei∫ən] - применение
brittle ['britl] - хрупкий, ломкий
car body - кузов автомобиля
constituent [kən'stitjuənt] - компонент
crack [kræk] - трещина
creep resistance - устойчивость к ползучести
definition [defi'ni∫ən] - определение
density ['densiti] - плотность
ductility [dʌk'tiliti] - ковкость, эластичность
failure ['feiljə] - повреждение
gradual ['grædjuəl] - постепенный
permanent ['pɜ:mənənt] - постоянный
rigid ['ridʒid] - жесткий
to sink [siŋk] - тонуть
square root ['skweə 'ru:t] - квадратный корень
stiffness ['stifnis] - жесткость
strain [strein] - нагрузка, напряжение, деформация
strength [stirəŋө] - прочность
stress [stres] - давление, напряжение
tensile strength - прочность на разрыв
toughness ['tʌfnis] - прочность, стойкость
yield strength [ji:ld] - прочность текучести
Young modulus - модуль Юнга
General understanding:
-
What is the density of a material?
-
What are the units of density? Where low density is needed?
-
What are the densities of water, aluminium and steel?
-
A measure of what properties is stiffness? When stiffness is important?
-
What is Young modulus?
6. What is strength?
7. What is yield strength? Why fracture strength is always great than yield strength?
8. What is ductility? Give the examples of ductile materials. Give the examples of brittle materials.
9. What is toughness?
10. What properties of steel are necessary for the manufacturing of:
a) springs,
b) car body parts,
c) bolts and nuts,
d) cutting tools?
11. Where is aluminium mostly used because of its light weight?
Exercise 1. Find the following words and word combinations in the text:
-
количество массы в единице объема
-
килограмм на кубический метр
-
мера сопротивления деформации
-
отношение приложенной силы на единицу площади к частичной упругой деформации
-
жесткая конструкция
-
прочность на сжатие
7. способность материала деформироваться не разрушаясь
-
поглощать энергию путем деформации
-
обратно пропорционально квадрату размера дефекта
-
постепенное изменение формы
-
повышенные температуры
-
высокие растягивающие усилия
Exercise 2. Translate into English the following:
1. Плотность измеряется в килограммах на кубический метр.
-
Большинство материалов имеют более высокую плотность, чем вода и тонут в воде.
-
Плотность материала очень важна, особенно в авиации.
-
Модуль Юнга - отношение приложенной силы к упругой деформации данного материала.
-
Чем более металл жесткий, тем менее он деформируется под нагрузкой.
-
Когда металл растягивают, он сначала течет, то есть пластически деформируется.
-
Свинец, медь, алюминий и золото - самые ковкие металлы.
-
Сопротивление ползучести является очень важным свойством материалов, которые используются в авиационных моторах.
WELDING
Welding is a process when metal parts are joined together by the application of heat, pressure, or a combination of both. The processes of welding can be divided into two main groups:
• pressure welding, when the weld is achieved by pressure and
• heat welding, when the weld is achieved by heat. Heat welding is the most common welding process used today.
Nowadays welding is used instead of bolting and riveting in the construction of many types of structures, including bridges, buildings, and ships. It is also a basic process in the manufacture of machinery and in the motor and aircraft industries. It is necessary almost in all productions where metals are used.
The welding process depends greatly on the properties of the metals, the purpose of their application and the available equipment. Welding processes are classified according to the sources of heat and pressure used.
The welding processes widely employed today include gas welding, arc welding, and resistance welding. Other joining processes are laser welding, and electron-beam welding.
Gas Welding
Gas welding is a non-pressure process using heat from a gas flame. The flame is applied directly to the metal edges to be joined and simultaneously to a filler metal in the form of wire or rod, called the .welding rod, which is melted to the joint. Gas welding has the advantage of using equipment that is portable and does not require an electric power source. The surfaces to be welded and the welding rod are coated with flux, a fusible material that shields the material from air, which would result in a defective weld.
Arc Welding
Arc-welding is the most important welding process for joining steels. It requires a continuous supply of either direct or alternating electrical current. This current is used to create an electric arc, which generates enough heat to melt metal and create a weld.
Arc welding has several advantages over other welding methods. Arc welding is faster because the concentration of heat is high. Also, fluxes are not necessary in certain methods of arc welding. The most widely used arc-welding processes are shielded metal arc, gas-tungsten arc, gas-metal arc, and submerged arc.
Shielded Metal Arc
In shielded metal-arc welding, a metallic electrode, which conducts electricity, is coated with flux and connected to a source of electric current. The metal to be welded is connected to the other end of the same source of current. An electric arc is formed by touching the tip of the electrode to the metal and then drawing it away.
Vocabulary
to join [dʒɔin] - соединять
pressure welding - сварка давлением
heat welding - сварка нагреванием
instead [in'sted] - вместо, взамен
bolting ['bəultiŋ] - скрепление болтами
riveting ['rivitiŋ] - клепка
basic ['beisik] - основной
to manufacture [mænju'fækt∫ə] - изготовлять
to depend [di'pend] - зависеть от
purpose ['рз:pəs] - цель
available [ə'veiləbl] - имеющийся в наличии
equipment [i'kwipmənt] - оборудование
source [so:s] - источник
gas welding - газосварка
arc welding - электродуговая сварка
resistance welding - контактная сварка
laser welding - лазерная сварка
electron-beam welding - электронно-лучевая сварка
flame [fleim] - пламя
edge [edʒ] - край
simultaneously [siməl'teniəsli] - одновременно
filler ['filə] - наполнитель
wire [waiə] - проволока
rod [rɒd] - прут, стержень
to melt [melt] - плавить(ся)
joint [dʒɔint] - соединение, стык
advantage [əd'vɑ:ntidʒ] - преимущество
to require [ri'kwaiə]
surface ['sз:fis ]
coated ['kəutid ]
flux [flΛks ]
fusible ['fju:zibl ]
to shield [∫i:ld ]
touching ['tΛt∫iŋ]
tip [tip ]
General understanding:
-
How can a process of welding be defined?
-
What are the two main groups of processes of welding?
-
How can we join metal parts together?
-
What is welding used for nowadays?
-
Where is welding necessary?
-
What do the welding processes of today include?
-
What are the principles of gas welding?
-
What kinds of welding can be used for joining steels?
-
What does arc welding require?
10. What is the difference between the arc welding and shielded-metal welding?
Exercise 1. Find the following words and word combinations in the text:
-
сварка давлением
-
тепловая сварка
-
болтовое (клепаное) соединение
-
процесс сварки
-
зависеть от свойств металлов
-
имеющееся оборудование
-
сварочный электрод
-
плавкий материал
-
дефектный сварной шов
-
непрерывная подача электрического тока
-
электрическая дуга
-
источник электрического тока
OTHER TYPES OF WELDING
Non-consumable Electrode Arc welding
As non-consumable electrodes tungsten or carbon electrodes can be used. In gas-tungsten arc welding a tungsten electrode is used in place of the metal electrode used in shielded metal-arc welding. A chemically inert gas, such as argon, helium, or carbon dioxide is used to shield the metal from oxidation. The heat from the arc formed between the electrode and the metal melts the edges of the metal. Metal for the weld may be added by placing a bare wire in the arc or the point of the weld. This process can be used with nearly all metals and produces a high-quality weld. However, the rate of welding is considerably slower than in other processes.
Gas-Metal Arc
In gas-metal welding, a bare electrode is shielded from the air by surrounding it with argon or carbon dioxide gas and sometimes by coating the electrode with flux. The electrode is fed into the electric arc, and melts off in droplets that enter the liquid metal of the weld seam. Most metals can be joined by this process.
Submerged Arc
Submerged-arc welding is similar to gas-metal arc welding, but in this process no gas is used to shield the weld. Instead of that, the arc and tip of the wire are submerged beneath a layer of granular, fusible material that covers the weld seam. This process is also called electro slag welding. It is very efficient but can be used only with steels.
Resistance Welding
In resistance welding, heat is obtained from the resistance of metal to the flow of an electric current. Electrodes are clamped on each side of the parts to be welded, the parts are subjected to great pressure, and a heavy current is applied for a short period of time. The point where the two metals touch creates resistance to the flow of current. This resistance causes heat, which melts the metals and creates the weld. Resistance welding is widely employed in many fields of sheet metal or wire manufacturing and is often used for welds made by automatic or semi-automatic machines especially in automobile industry.
Vocabulary:
gas-tungsten - сварка оплавлением вольфрамовым электродом в среде инертного газа
inert [i'nɜ:t] - инертный
edge [edʒ] - край
bare [bеə] - голый
rate [reit] - зд. скорость
gas-metal arc - аргоно-дуговая сварка
considerably [kan'sidərəbli] - значительно, гораздо
surrounding [sə'raundiŋ] - окружающий
carbon dioxide ['кɑ:bən dai'ɒksaid] - углекислый газ
droplet ['drɒplit] - капелька
liquid ['likwid] - жидкость, жидкий
beneath [bi'ni:ө] - под, ниже, внизу
layer ['leiə] - слой
weld seam [si:m] - сварной шов
resistance - сопротивление
clamp [klæmp] - зажим, зажимать
sheet [∫i:t] - лист
fusible ['fju:zəbl] - плавкий
granular ['grænjulə] - плавкий
semi-automatic [semi ,ɔ:tə'mætik] - полуавтоматическая
to create [kri:'eit] - создавать
to submerge [səb'mɜ:dʒ] - погружать
General understanding:
-
What is the difference between the arc-welding and non consumable electrode arc welding?
-
What are the disadvantages of the non-consumable electrode arc welding?
-
How is electrode protected from the air in gas metal arc welding?
-
What is submerged arc welding?
6. What is the principle of resistance welding? 6. Where is semi-automatic welding employed?
Exercise 1. Translate into English:
-
вольфрамовый электрод
-
инертный газ
-
окисление
-
высококачественный сварочный шов
-
скорость сварки
-
аргон, гелий, углекислый газ
-
жидкий металл
-
слой плавкого материала в виде гранул
-
листовой металл
10. полуавтоматические сварочные станки
Exercise 2 Translate into Russian:
-
In resistance welding, heat is obtained from the resistance of metal to the flow of an electric current.
-
The heat from the arc melts the edges of the metal.
-
A bare electrode is shielded from the air by surrounding it with argon or carbon dioxide gas.
-
Submerged-arc welding is similar to gas-metal arc welding.
-
Electrodes are clamped on each side of the parts to be welded.
-
Resistance causes heat which melts the metals and creates the weld.
CORROSION OF METALS AND ALLOYS
Almost all metals and alloys subject to the action of atmospheric air or other surrounding media (for example, sea water, soil, acid and alkali solutions, organic liquids, etc.) are gradually destroyed, beginning from the surface, and lose their initial appearance. This progressive destruction of a metallic surface exposed to an external aggressive (active) medium is called corrosion.
Experience show that corrosive destruction depends mainly upon the following 3 factors: 1) the chemical nature of the metal or composition of the alloy and their structures; (2) the chemical nature of the surrounding medium and the percentage of aggressive matter in metals (oxygen, moisture, acids, alkalis, etc.) and (3) the temperature of the surrounding medium.
As to its character, metal corrosion may be classified as (1) uniform corrosion, in which the whole surface of the metal or alloy is corroded with equal intensiveness, (2) localized corrosion, in which only certain areas of the surface are attacked; (3) selective corrosion, where only separate structural components of an alloy are effected and (4) intercrystalline corrosion, which involves destruction of the metal or alloy along its grain boundaries.
Vocabulary:
destruction [dis'trΛk∫ən] - разрушение
chemical nature ['kemikel 'neit∫ə] - химическая природа
uniform corrosion ['ju:nifɔ:m kə'rəuƷn] - равномерная коррозия
localized corrosion ['ləuk(ə)laizd] - местная коррозия
selective corrosion [si'lektif] - избирательная коррозия
intercrystalline corrosion- межкристаллитная коррозия
General understanding:
-
What is corrosion?
-
What does the corrosive destruction depend on?
-
What types of corrosive destruction do you know?
Exercise 1. Retell the text.
TYPES OF CORROSION
Oxidation. The conversion of the surface portions of metal to oxide on heating in air or oxygen is generally termed « oxidation». Oxidation is regarded as a type of corrosion.
Other special types of corrosion. Generally corrosion starts on the surface of a metallic specimen. If a large portion of the surface is affected, it is said to be «general corrosion» if only small area it is called «localized corrosion», if confined to small points, so that definite holes are produced, we speak of pitting. If corrosion produces grooves following grain-boundaries, or perhaps (on a much large scale) following the waterline on a partly immersed metallic platter, we speak of «grooving». Grooves may also be developed along the line, where two dissimilar metals meet, or in zones running parallel to the surface to a weld. If in rolled or extruded metals corrosion extends along certain planes parallel to the surface, it is called «layer corrosion». Under atmospheric conditions, the voluminous corrosion product formed along these planes may lever the intervening layers apart, so that, the material divides into flakes; this is known as «foliation».
Vocabulary:
oxidation [ɔksi'dei∫(ə)n] - окисление
general corrosion['dƷen(ə)rəl] - общая коррозия
localized corrosion ['ləuk(ə)laizd kə'rəuƷn] - местная коррозия
layer corrosion ['leiə(r)] - коррозия слоя
General understanding:
-
What is oxidation?
-
What are other special types of corrosion?
Exercise 1. Describe a type of corrosion which you saw in the practice.
CORROSION PROTECTION
Part 1
Everybody knows how widely steel and iron products are used. But almost in all applications regardless of the environment, these products are coated by some means in order to prevent corrosion and make possible lasting beauty. Among different types of coatings organic coatings comprising paints, lacquers and varnishes are most widely used. Organic coatings protect steel from corrosion by forming a corrosion-resistant barrier between steel and corrosive environment, but corrosion protection offered by the barrier film is only as good as the corrosion resistance of the film itself. There is no protection at the discontinuities of the film. So in some cases additional protective coatings reinforce organic coatings.
Next widely used coatings are metallic coatings, which can be divided into 2 groups. Metals such as zink, cadmium and magnesium under most conditions are anodic to steel. The degree of protection depends on the corrosion resistance of the coating metal and its thickness. The second group includes metals catholic to steel such as lead, thin, nickel, chromium, which protect it as a corrosion barrier. The degree of protection depends on the corrosion resistance of the coating metal and its continuity.
Ceramic coating commonly known as porcelain (or vitreous) also protects large tonnages of steel. It has exceptional corrosion and neat resistance and is available in a variety of colour.
Vocabulary:
сoat [kəut] - покрывать
coating ['kəutiŋ] - покрытие
corrosion resistance [kə'rəuƷn ri'zistəns] - коррозийная стойкость
film [film] - пленка
discontinuity[diskən'tinju:iti] - нарушение цельности
vitreous ['vitriəs] - стекловидный
Part 2
Sizable amounts of steel are protected by lead -tin alloys and aluminium. Smaller quantities of steel are protected by other special metals and alloys. Gold and tungsten, for example, could be good protective metals. Composite coatings are also important in protection of steel surface. These may contain more than two components.
AVAILABLE COATING METHODS. Because of natural instability of the steel surface, it is readily contaminated by oxides, rust, or oils used for rust prevention during transportation and storage. To prepare the steel for satisfactory application of a protective coating, the surface must be freed of undesirable contaminants, the degree of cleanliness required depending on the characteristics of the protective coating to be applied. Acid pickling for removal of scale and oxides and alkali decreasing for removal of oil and lubricants are the most common processes to be used as surface cleaning methods.
Vocabulary:
rust [rΛst] - ржавчина
lubricant ['lu:brikeit] - смазка
contaminate [kən'tæminit] - загрязнять
apply [ə'plai] - наносить, применять
pickling [pikliŋ] - травление
Exercise 1.Answer the following questions:
-
What means are most widely used to prevent corrosion?
-
What does the degree of protection depend on?
-
What coating methods are available?
Глоссарий
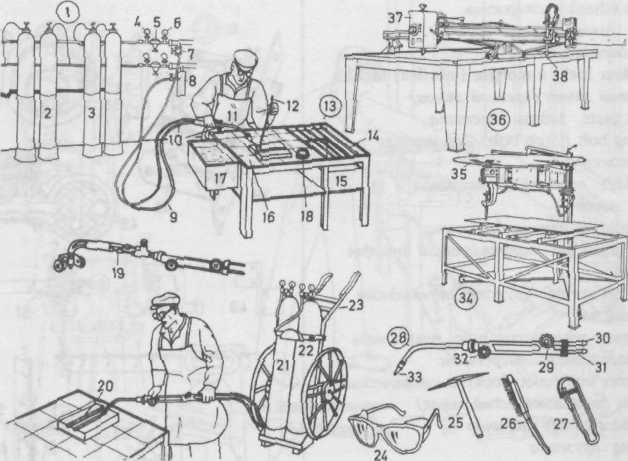
Gas Welder Газосварщик

-
Gas cylinder manifold батарея газовых баллонов
-
acetylene cylinder баллон с ацетиленом
-
oxygen cylinder баллон с кислородом
-
high-pressure manometer манометр высокого давления
-
pressure-reducing valve (reducing valve, pressure regulator) редуктор
-
stop valve запорный вентиль
-
hydraulic back-pressure valve for low-pressure installations гидравлический обратный клапан для аппаратов низкого давления
-
gas hose газовый шланг
-
oxygen hose шланг для подачи кислорода
-
welding torch (blowpipe) сварочная горелка
-
welding rod (filler rod) присадочный пруток
-
welding bench сварочный стенд (стол)
-
grating решётка
-
scrap box ящик дли отходов
-
bench covering of chamotte slabs покрытие сварочного стола шамотовыми плитками
-
water tank ванночка с водой
-
welding paste (flux) паста для сварки (флюс)
-
welding torch (blowpipe) with cutting attachment and guide tractor газовая горелка с резаком и ходовым колесиком
-
welding torch (blowpipe) with cutting attachment and guide tractor газовая горелка с резаком и ходовым колесиком
-
oxygen cylinder баллон с кислородом
-
acetylene cylinder баллон с ацетиленом
-
cylinder trolley meлeжкa для перевозки газовых баллонов
-
welding goggles защитные очки
-
chipping hammer обрубочный молоток
-
wire brush проволочная щётка
-
torch lighter (blowpipe lighter) зажигательное устройство (запальник)газовой горелки
-
welding torch (blowpipe) сварочная горст
-
oxygen control регулятор подачи кислорода
-
oxygen connection патрубок для присоединения кислородного баллона
-
gas connection (acetylene connection) патрубок для присоединения газового (ацетиленового) баллона
-
gas control (acetylene control) регулятор подачи ацетилена
-
welding nozzle сопло горелки
-
cutting machine газорезательная машина
-
circular template круглое (криволинейное) лекало (шаблон)
-
universal cutting machine универсальная машина кислородной резки
-
tracing head копировальная головке
-
cutting nozzle сопло резака
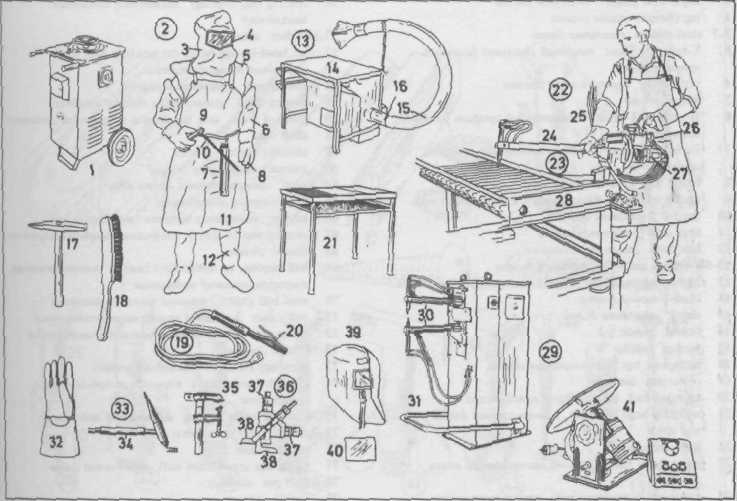
Arc Welder Электросварщик

-
welding transformer сварочный трансформатор
-
arc welder шлем электросварщика
-
arc welding helmet откидной (верх) козырёк смотрового окошечка (смотрового отверстия) [шлема электросварщика]
-
flip-up window защитное стекло
-
shoulder guard защитный наплечник
-
protective sleeve защитный нарукавник
-
electrode case футляр с электродами
-
three-fingered welding glove перчатка сварщика с тремя пальцами
-
electrode holder электрододержатель
-
electrode электрод
-
leather apron кожаный фартук
-
shin guard защитный наколенник
-
welding table with fume extraction equipment рабочий стол сварщика с вытяжным устройством
-
table top верхняя крышка рабочего стала
-
movable extractor duct гибкий отсасывающийй шланг
-
extractor support патрубок для присоединения отсасывающего шланга
-
chipping hammer обрубочный молоток
-
wire brush проволочная щётка
-
welding lead сварочный провод
-
electrode holder электрододержатель
-
welding bench сварочный стол (верстак)
-
spot welding точечная сварка
-
spot welding electrode holder электрододержатель для точечной сварки
-
electrode arm плечо
-
power supply (lead) токоподающий провод
-
electrode-pressure cylinder цилиндр(масляный) для сжатия электродов
-
welding transformer сварочный трансформатор
-
workpiece свариваемая конструкция
-
foot-operated spot welder машина для точечной сварки с ножным управлением
-
welder electrode arms рычаги, поддерживающие сжимаемые электроды
-
foot pedal for welding pressure adjustment ножная педаль для зажатия электродов
-
five-fingered welding glove перчатка сварщика с пятью пальцами
-
inert-gas torch for inert-gas welding (gas-shielding arc welding) горелка для дуговой сварки в защитной среде инертного газа
-
inert-gas (shielding- gas) supply подача инертногоо газа
-
work clamp (earthing clamp) зажим для подключения заземляющего провода (проводника)
-
fillet gauge (Am. Gage) (weld gauge) [for measuring throat thickness)] шаблон для контроля формы (поперечного сечения) сварного шва
-
micrometer микрометр
-
measuring arm измерительное плечо
-
arc welding helmet защитная маска электросварщика
-
filter lens смотровое стекло (шлема)
-
small turntable
Литература
-
Андреева Г.Я., Гуаль Л.Л., Лев А.Л. Сборник технических текстов на английском языке. - М. 2013г.
-
Чистик М.Я. Учебник английского языка для политехнических ВУЗов. - М. 2010г.
-
David Bonamy "English for Technicals Students".


